Terrible weather. Cold, strong winds, heavy rain...
So back to MUSTANG SCIENCE!
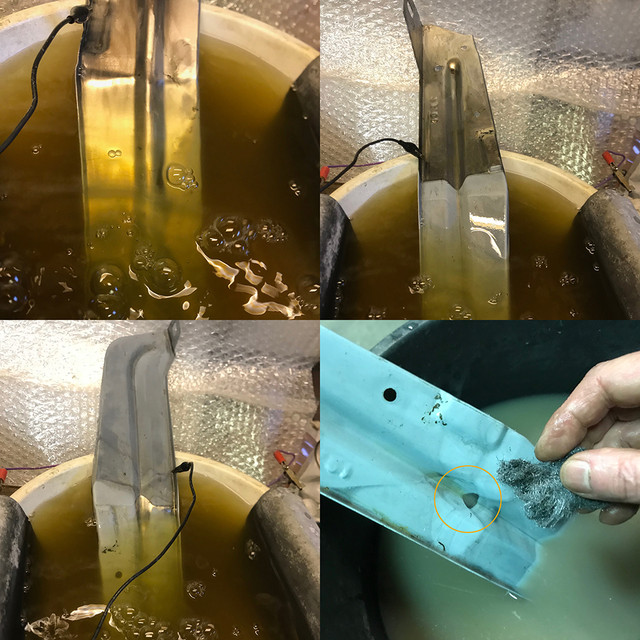
Started with retrying to get copper on zamak...

When nothing works: just like for welding, Dr Oetker is your best friend...

Because zamak (pot metal) is an alloy mostly composed of zinc. Its reacts the moment you submerge it with acid. Once you add power, even low, you plate and remove at the same time. Explains the brass layer result of last week.
I've red on the subject and pro's are using cyanid baths for this. Short story, no way I'm gonna go that way. Instead wanted to see what I could do with mild alkalin bath using the above baking soda.
And what do you know: it works!
However, because I wanted to try and see first, I didn't have a big enough bath. less than 1/2 liter or something. To get a useful layer on this part in one pass, I'd need make more and let it over night at very low voltage, may be 1 volt to get a much thicker layer that I can use to buff the old pitting. That would also prevent burns and give an even deposit as seen on the pict caused at where the part was submerge and then exposed to the air. If weather is bad again next week, I might try that. No rush on this. But if you compare with past post pictures, it's now really copper vs brass. If the surface would be perfect, its kinda beautiful too. Amazing what 5 tea spoons of baking soda can do


Its been a while since I've showed you horror pictures, so as weather might be "paint ok" in a not too far future, I've collected new parts, enough material for dirty work!

Some will be zinc plated, some painted and some both. So removed a few more parts on the car. Notice the rusty hardware, and started by spreading some paint remover on the latch mechanism..

Here some horror picts after 16 hours. But after a good cleaning massage, things started look at some mustang part.

Did the other part (under a new layer of remover right now) and because the lady rust is at work on this latch too, its now in my derust bath, and will stay prolly 48 hours in it.

The light bezels were also derusted. A bit strange part, looks like stainless, but there is some rust.
I'll return on these, as one has some damage and need make a buck to hammer it back to a round shape.
I'll need to weld or solder one of the lip too, it broke once I've turned the screw on the car. Anyone repaired one of these?

In between, been busy cooking the hardware on saturday and electro cleaned them today for a couple of hours.

For these, I've used the receipt found last week that deposits a strong and thick copper layer.
Left, raw from electro cleaning, in the middle after being plated, on the right, just brushing them with wool makes the copper shine like a new penny.
If you wonder why copper plate them and not directly apply nickel... Unlike zinc that will corrode to death to protect the underlaying substrate by its negative charge. Nickel is the opposite. The substrate will corrode to protect the nickel. Having the copper in between reduces this behaviour a lot.

I did not polish any of the hardware, just brushed them and plated them in copper and then they received a layer of nickel. I'll polish some if that will be required later on but for now the 50 years old latch hardware is good to go for another 1/2 century.
I've started other stuffs, and should have more horror picts to show soon

To be continued...