Misery weather...
As its a new year, its time to try something new: time for MUSTANG SCIENCE!!


As said in prev post, past weeks I've been busy reading on plating and decided to give a it a try.
And this week, received my metal rods, all 99,xx something pure. I haven't checked if true and would not even know how to tell, except that all articles on the subject were clear on that point. If the used metals aren't pure, the expected color of the electrolysis will have a different coloration. Green for nickel, blue for copper and greyish transparent for zinc.
Just to be clear before continue: I have zero idea about what i'm talking about! Except I've eaten PDF's, red articles and seen tons of videos on the subject for the past month. The goal is first to see if I can really plate at home, get the kind of finish you'd expect to see on car parts, and if this is doable regarding costs and safety.
I just want get rid of the rust and get the bling bling back that painting only will never give back.

I started first with nickel. Not because its better than copper or zinc. Its just the part i've prepped to be first victims, would look pretty with nickel.
The first goal is to prep some solution, called nickel acetate.
There are companies offering some cristals, that you can dilute in distilled water, giving you a ready to use solution.
Fine, but what is the fun in that??? Plus, may the "from scratch" fail, I can always turn to these options later on.
So in case you wonder how that goes: its really easy. Take some distilled white vinegar (I usually use another more agressive one for rust, but its having added chemicals i don't want here)
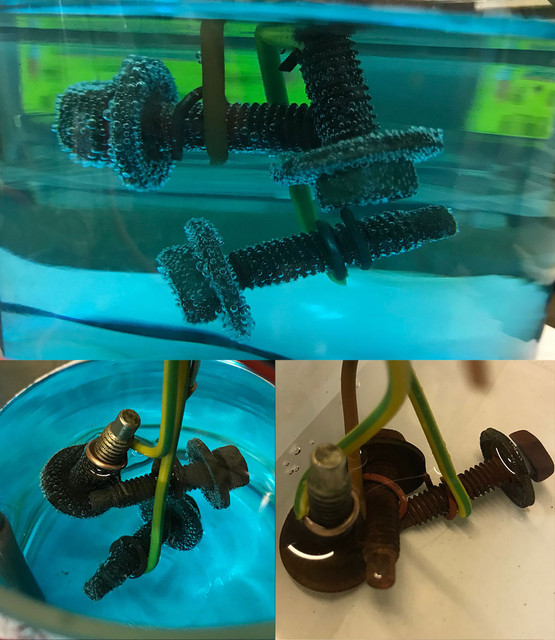
Add a tea spoon of salt to increase the liquid conductivity. You need 2 electrodes to prep this juice. Thats why I've ordered a pair rods of each metals. Just connect each one to some power source, a phone charger or anything under 6v will do just fine. Then its a matter of waiting...
The same can be done with the 3 metals.
On the pict above, the small ones show from 1 hour (almost nothing) up to 8 hours (nice and clear green) and on the larger pict, the solution after 24 hours.
I was already very happy with this result, as it's not only the expected green for nickel, its also nicely saturated. It will continue to saturate and become darker as I plate stuff (and do not contaminate it) , but to plate something correctly, you need a good concentration to get nice even results. The darker the solution, the shorter the plating time too.

I do Mustang stuffs, so not planning to plate some coin or some grandma's ring as seen on many utube vids. If I can't plate a part like this hood latch right away, it's useless. So lets find out...
The part was really rusty and de-rusted during past weeks, and gave it to prevent contamination a good cleaning/degreasing and metal brushing, followed by a 1 minute bath in chloridric acid. (you can see on the pict after a few secs how that stuff is corrosive). Recomended in many articles, as it gets rid of the last bits of potential oil or grease and etches the surface. The one minute over, it went to another bath to be rinced with distilled water.
Then the unprepared me (need buy some copper wire and many other details) hooked the part so it was fully submerged and instead of using one rod, I've used them both, on each side (connected to each other). This time, they received the positive and the part itself the negative. It's said on many articles, the curent is set by dividing one amp by the surface of the to be plated object. My power source being totally new to me, I couldn't set it up as I wanted, so went by the trick given by many: best looking and durable results are obtained with the lowest voltage with little to no bubbles. There are too big bubbles on the above pict, this was corrected after the pict was taken...

Based on some graph with power, acidity and surface (that I've totally ignored in practice

), I've let the baby exactly 2 hours into the solution, stopped at 1 hour to change the orientation of the part. So it would be facing or be near of the sacrificial rods.
I forgot take a pict before (only in acid).
One thing is clear: it works! yeah!

The other clear part is that the result is defined by the polishing work. In this case, even if the small dents are magnified by the extra bright light i was holding to make the picts, it shows I did a poor job and wire wheel is far from being enough.
Also, using some polish paste did not remove any of the plating, meaning nickel really became the part and its not some layer that can be wiped away just like that. Something I was afraid of.
Even as is, its day and night compared to how it looked not 3 weeks ago. The right side of the pict, you can see the difference in 'blingblingness" with the other de-rusted bolts. It was having the exact same color/brightness.
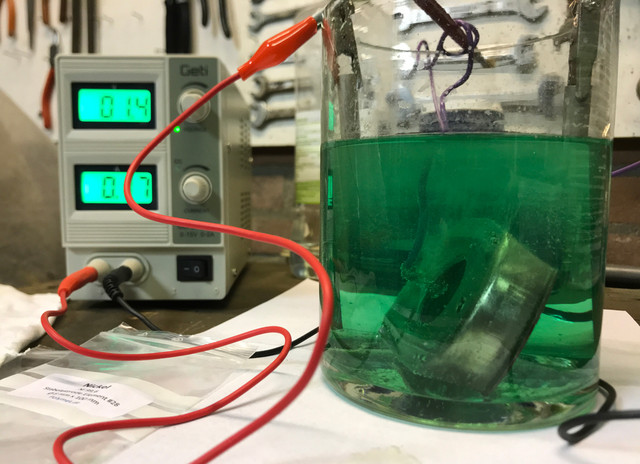
Totally electrified with the bling bling, while waiting, prepped another victim, one of the bracket holding the hood locks. Just like the hatch, I did not really polish much, and to see the effect, I did nothing (polishing/brush wise) on purpose to the inside to see how far you must go into the preparation.

Because of the result of the latch, I wanted to see how that goes with a bigger part (surface wise) and the amount of deposit I could get after 1 hour extra. On left side, the other hood lock, on the right the plated result.
While the surface of the outside is ok (and showed again, that a poorly polished surface results in the exact same surface result), the inner side was practically not plated.
I could not understand why...

...till it was time to clean up. As I do not have yet solid/permanent connections and propper materials, it was clear that the second rod, supposed to be the positive was not having a good connection, if any. So the inner side received a much lower amount of nickel, not being exposed directly to a rod.
All with all 2019 starts very bright! Pretty happy with the results. No need to say that both will be repolished and get a new coating, but this first taste at home plating showed me that, at least for nikkel, its perfectly doable and easy, that good quality can be obtained once you have bits of experience and its very affordable. By the look of the rods, I know I can plate tons of parts before i need order a new one (15 euros). I also think I will later on invest in coils/wires and sheets, to ensure best coverages per parts.
Next step will be to buy containers to store these solutions, as once done, they can be reused over and over. And start think of making a bigger bath to handle multiple parts in one pass and also allow bigger parts...
To be continued...